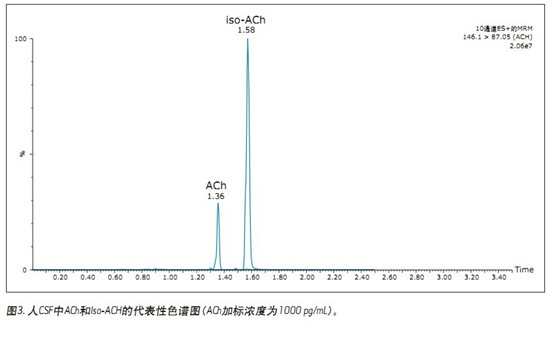
UPLC/MS/MS quantitative assay for simultaneous quantitative analysis of acetylcholine, histamine and its metabolites in human cerebrospinal fluid (CSF) using CORTECS UPLC HILIC columns to improve peak resolution, sensitivity and speed of analysis Application Benefits ■CORTECSTMUPLC® HILIC column resolution enables simultaneous quantification of ACh, HA and its metabolites ■Total run time is only 2.5 min, meeting high throughput screening requirements ■96-well plate application simplifies sample Preparation (less than 15min) Waters Solutions Keywords <br> bioanalysis, biomarkers, cerebrospinal fluid, quantitative, neurotransmitter, acetylcholine, choline, histamine, T-methylhistamine, T-methylimidazolium acetate, UPLC, HILIC, CORTECS Introduction <br>In drug discovery, biochemical markers from body fluids are often used as an effective means of diagnosing disease and assessing drug efficacy. Acetylcholine (ACh) and histamine (HA) are highly polar neurotransmitters found in almost all mammalian tissues. They play a role in sleep regulation 1-3, memory, learning, and immune response. Brain ACh levels will lead to decreased memory function disorders, especially Alzheimer's patients ACh supply is obviously insufficient 1. Therefore, extensive research has been conducted on how to promote the release of ACh in the treatment of such cognitive disorders. Histamine is produced by the decarboxylation of histidine. Histamine is either stored or rapidly inactivated by its major degrading enzyme histamine-N-methyltransferase or diamine oxidase and metabolized into two major metabolites, t-methylhistamine (t- mHA) and τ-methylimidazoliumacetic acid (t-MIAA). Histamine in the brain is involved in a wide range of physiological functions such as sleep-wake cycle regulation, arousal, appetite control, cognition, learning and memory 2,3 . It is understood that the histamine metabolites in the cerebrospinal fluid of patients with schizophrenia increase 4,5 . Therefore, the concentrations of HA, t-mHA and t-MIAA in cerebrospinal fluid (CSF) can be used as indicators of brain histamine activity. In view of the important physiological role of ACh, HA and their respective metabolites, the measurement of physiological concentration changes can be used as an indicator for disease progression or drug treatment. This function makes these substances a very important biochemical marker in drug development. . The development of targeted assays for ACh, HA, and their metabolites is a constant challenge because it requires not only high sensitivity and selectivity, but also faster sample analysis. In addition, there is a large difference in endogenous circulating concentrations between ACh, HA and their respective metabolites, such that simultaneous determination of these materials requires a broad quantitative dynamic range. Although there are a variety of methods for quantifying such analytes (GC/MS, HPLC-EC and LC/MS) 6-8 , there are few methods for simultaneous determination of rodent brain microdialysis fluid or CSF matrix. ACh, HA and their respective metabolites 9 . In the elution conditions with higher aqueous phase, the analyte and matrix inhibitory components may co-elute and the MS ionization ability is poor, so that the reverse phase (RP) liquid chromatography combined with MS detection method is less sensitive. Limited by 10 . Hydrophilic liquid chromatography (HILIC) can improve the chromatographic retention of polar substances. It can be orthogonalized with reversed-phase chromatography for the separation of polar and ionizable compounds, and can improve the mass spectrometry response. The best choice for polar analyte separation challenges. The methods described herein demonstrate simultaneous quantification of ACh, HA and their respective metabolites in human CSF in 96-well plates. Samples were prepared using a one-step sample preparation technique, and 20 μL of the sample was diluted for further analysis by HILICUPLC/MS/MS. The high-efficiency CORTECS UPLC HILIC column with sub-2μm particles enables separation of analytes from endogenous matrix and improves analytical sensitivity. The analysis time is only 2.5min, so the method is fast, sensitive, accurate and selective, in line with drug development. High-throughput bioanalytical needs. experiment UPLC Condition <br>System: ACQUITY UPLC UPLC separation In this application note, we performed chromatographic separation of ACh, HA, and their respective metabolites using a sub-2μmCORTECS UPLC HILIC column and an ACQUITY UPLC system. The optimum ammonium formate concentration (mobile phase A) was determined to be 100 mM (pH 3) and mobile phase B consisted of acetonitrile. At an optimum flow rate of 0.5 mL/min and a gradient of 30%-90% B, the analysis cycle time was 2.5 min at a column temperature of 45 °C. The concentration of the mobile phase additive has a significant impact on method specificity, sensitivity, and ability to chromatographically separate the analyte of interest from the mobile phase and the endogenous matrix. A higher buffer concentration of 100 mM (instead of 20 to 50 mM) greatly improved the peak width of the post-eluting analyte. In particular, ammonium citrate with a high mM concentration significantly reduced the peak tailing of t-MIAA. ACh, Ch and their respective metabolites in the lower limit control standard (LLQC), chromatographic retention time and performance are shown in Figure 2. ACh, Ch and their respective metabolites and the isomer of ACh, Iso-ACh(3-carboxypropyl)trimethylammonium, eluted within 1.35 to 1.75 min with a baseline peak width of less than 3.7 s. Since Iso-ACh(3-carboxypropyl)trimethylammonium is present in higher concentrations in the brain, it was analyzed in this study. It is reported to be a substrate for the enzyme (G-betaine hydroxylase) in carnitine biosynthesis11. ACh and Iso-ACh are isomers, and it is important to ensure the chromatographic resolution of ACh when performing accurate quantification of ACh in CSF. In human CSF sample extracts (Figure 3), the presence of high concentrations of endogenous Iso-ACh was highlighted. ACh was spiked into human CSF at a concentration of 1000 pg/mL to demonstrate chromatographic separation and concentration differences between ACh and Iso-ACh. Sample Preparation <br> Artificial CSF (aCSF) was chosen as an alternative matrix for the preparation of a standard curve to quantify all analytes. aCSF is easier to obtain and does not cost as much as human CSF, so it is more practical to use it as a substitute matrix. To ensure that aCSF can be used as a suitable matrix for accurate quantification of all analytes, we prepared spiked human CSFQC samples of ACh, HA and t-mHA, and aCSFQC samples of Ch and t-MIAA. The QC samples of Ch and t-MIAA are formulated by aCSF because of the high concentration of ng/mL of endogenous substrate levels in human CSF, making it difficult to quantify in a linear range suitable for other analyte determinations by standard addition methods. Therefore, QC samples of Ch and t-MIAA were prepared using aCSF. In addition, the concentration of Ch in human CSF is higher, so it is necessary to dilute the sample with aCSF for accurate quantification within the dynamic detection range. As described by Zhang, Tingley, Tseng et al., all human CSFQC samples and human CSF samples contained a final concentration of 4 mM stabilizer physostigmine to prevent the enzymatic conversion of ACh to Ch and acetate. Standard curves, human CSFQC samples, aCSFQC samples and samples were prepared using 1 mL 96-well plates. One-step sample dilution (sample amount: acetonitrile amount 1:5) was carried out by adding acetonitrile containing the internal standard described in the experimental section. Sample preparation can be completed in 15 minutes using a 96-well plate, meeting the high-throughput analysis requirements required to develop quantitative analytical methods. Linearity, Accuracy, and Accuracy <br>Quantitative analysis using Deuterated Stable Isotope Labeling Standards (ISTD) from ACh, HA, t-mHA, and t-MIAA. Ch quantification was performed using the d4ACh internal standard. The standard curves (1/x weighting) of ACh, Ch, HA, t-mHA and t-MIAA are linear (>0.99) within the quantitative dynamic range. The dynamic range, linearity, average QC accuracy, and lower limit of quantitation (LLOQ) of each analyte are shown in Table 2. A representative calibration curve for ACh is shown in Figure 4. QC samples were prepared from a mixture of human CSF and aCSF for evaluation of accuracy and reproducibility within the sample. Basal levels of ACh, HA and t-mHA were determined by analysis of unspiked human CSF. The QC concentration was determined by subtracting the average substrate level concentration from the CSF source to obtain the corresponding QC level. Representative results of spiked human CSFQC samples of ACh, HA and t-mHA, and spiked aCSFQC samples of Ch and t-MIAA are shown in Tables 3 and 4. After quantification of aCSF and human CSF calibration, the endogenous substrate concentration of each analyte in human CSF was determined using the aCSF standard curve. The basal level average measurements of ACh, Ch, HA, t-mHA, and t-MIAA are summarized in Table 5. Accuracy and accuracy values ​​are in compliance with FDA regulatory standards for LC/MS/MS testing12,13 . in conclusion references Febuxostat Intermediate,Febuxostat Api,Febuxostat-24 Intermediate,Febuxostat Ethyl Ester Shandong Bolode Bio-technology Co., Ltd. , https://www.bldpharma.com
MaryE.Lame, ErinE.Chambers and KennethJ.Fountain
Waters Corporation (Milford, MA, USA)
■The highly sensitive Xevo® TQ-SMS allows for smaller sample volumes and higher dilution ratios than previous reports
ACQUITY UPLC® System
Xevo TQ-S mass spectrometer
CORTECS UPLC HILIC column
ACQUITY® Collection Board 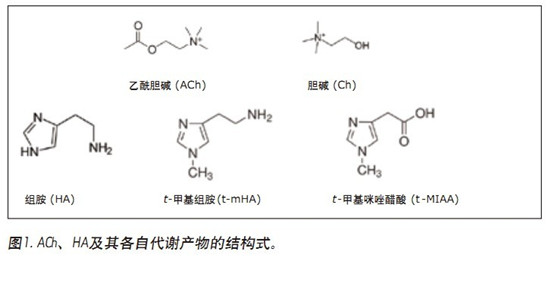
Sample Preparation
20 μL of artificial cerebrospinal fluid (aCSF) standard, aCSF quality control (QC) sample or human cerebrospinal fluid (containing 4 mM physostigmine) was transferred to a 1 mL 96-well plate. 100 μL of acetonitrile containing d4-acetylcholine, d4-histamine, d3-t-methylhistamine and d3-t-methylimidazoliumacetic acid (used as internal standard (ISTD)) at a concentration of 1 ng/mL, respectively, was added to the sample. . Cover the 96-well plate and mix it by vortexing. Then, the sample was centrifuged at 4000 rpm for 5 min, and 10 μL of the sample was taken for UPLC/MS/MS analysis.
Column: CORTECS UPLC HILIC 1.6μm, 2.1mmX100mm (part number 186007106)
Mobile phase A: 100 mM ammonium formate, pH 3
Mobile phase B: acetonitrile gradient: initial 90% mobile phase B, linearly increased to 60% in 0.75 min, held for 0.25 min, then linearly reduced to 30% in 0.25 min and held at 0.65 min. Finally return to the initial conditions within 1.9min.
Flow rate: 0.5mL/min
Column temperature: 45 ° C
Sample temperature: 6 ° C
Injection volume: 10μL
Running time: 2.5min
Collection plate: Waters® ACQUITY 1mL collection plate
MS Condition <br>Mass Spectrometer: Xevo TQ-S
Ionization mode: ESI+
Capillary voltage: 3.0kV
Desolvation gas temperature: 550 ° C
Cone gas flow rate: 150L / h
Desolvent gas flow rate: 900L/h
Collision chamber pressure: 3.58X10 (-3) mbar
Collision energy: optimized by component, see Table 1
Taper hole voltage: optimized by composition, see Table 1
Data management <br> Chromatography: MassLynx® software Quantitative: TargetLynx ™ software
Results and discussion
Mass Spectrometry <br>ACh, HA and their respective metabolites were detected and quantified using the Waters Xevo TQ-S mass spectrometer in ESI-MS/MS positive ion mode. The multiple reaction monitoring (MRM) channels selected for ACh, HA and their respective metabolites and corresponding deuterated stable isotope labeling forms (as internal standards) were automatically optimized using MassLynx IntelliStartTM software, as shown in Table 1. Each MRM channel has a dwell time as short as 25ms, and the MS system's fast scan time allows it to simultaneously acquire all compound peaks and reach >/=10 per peak data point. 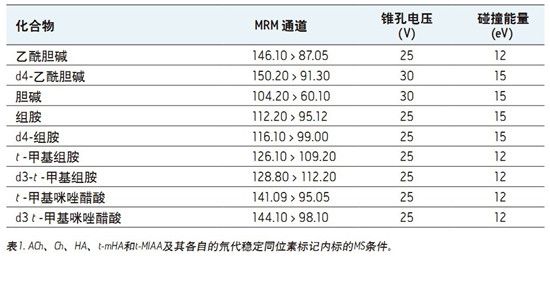
ACh, Ch, HA, t-mHA, and t-MIAA are strongly polar small molecule compounds present at relatively low concentrations, making it difficult to detect by conventional reverse phase chromatography. Further, since the related molecules (e.g., inner salts or other low molecular weight components derived) induction of matrix interference and co-elution of ion suppression, lead to biological matrix of these endogenous analytes are often more difficult to quantify 9 . 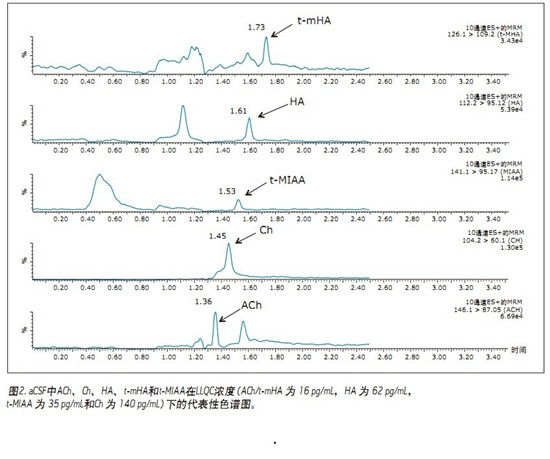
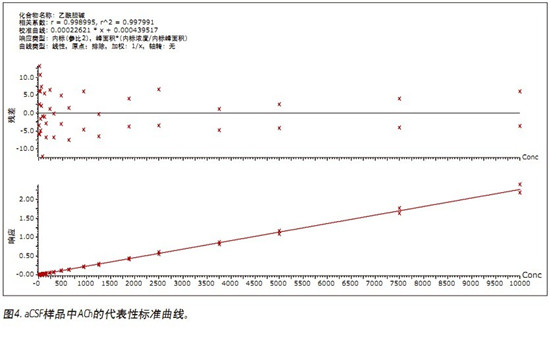
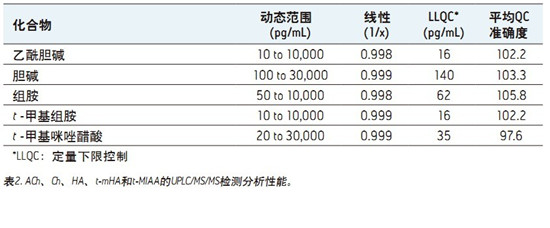
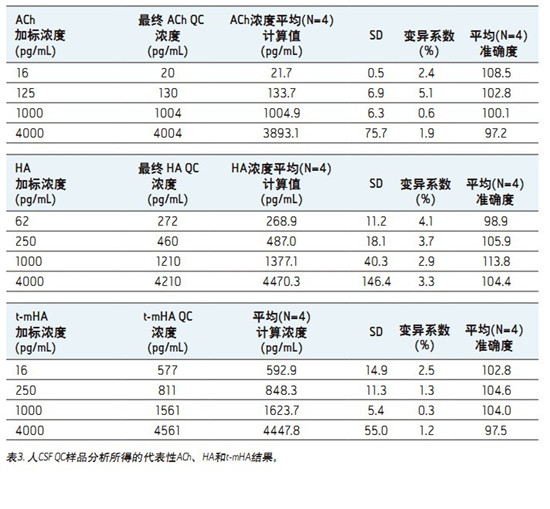
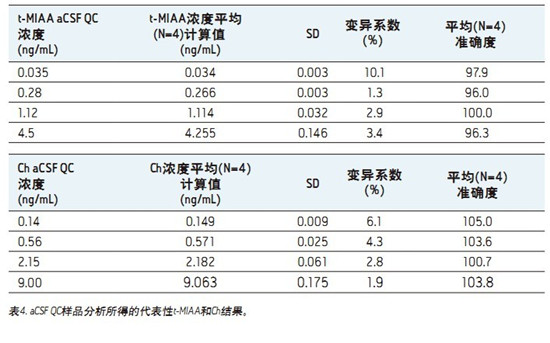
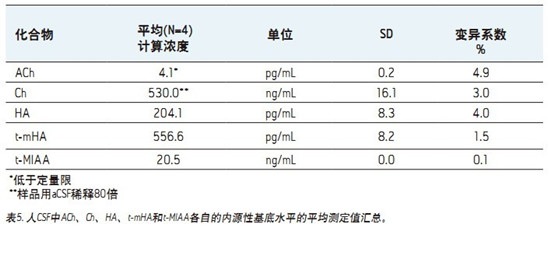
â– This fast, simple, sensitive and highly selective UPLC assay separates and simultaneously quantifies ACh, HA and their respective metabolites in human CSF.
â– Sample preparation can be completed in 15 minutes using a 96-well plate, providing the high throughput required for analytical methods in the R&D phase.
â– The resolution and sensitivity of the CORTECS UPLC HILIC column improves the separation and resolution of endogenous matrix components with an analysis time of only 2.5 min.
■The high sensitivity of the Xevo TQ-SMS allows detection limits as low as 10 pg/mL with a small sample (20 μL) and a 5-fold dilution with a wide quantitative dynamic range of 10-30 000 pg/mL.
â– The QC sample analysis results of all analytes are in compliance with FDA regulations, with a coefficient of variation of 0.3%-10.1% and an accuracy range of 94.7%-113.7%, indicating that the method is reproducible and accurate.
â– The methods presented in this paper demonstrate the potential for high selectivity and high sensitivity quantification of multiple neurobiomarkers during the development phase of drug development.
1. Jia JP, et al. Differential acetylcholine and choline concentrations in the cerebrospinal fluid of patients with Alzheimer's disease and vascular dementia. Chin Med J (Engl). 2004; 117(8): 1161-4.
2. Vohora D, Bhowmik M. Histamine H3 receptor antagonists/inverse agonists on cognitive and motor processes: relevance to Alzheimer's disease, ADHD, schizophrenia, and drug abuse. Front Syst Neurosci. 2012; 6: 72.
3. Jadidi-Niaragh F, Mirshafiey A. Histamine and histamine receptors in pathogenesis and treatment of multiple sclerosis. Neuropharmacology. 2010; 59(3): 180-9.
4. Ito C. The role of the central histaminergic system on schizophrenia. Drug News Perspect. 2004; 17(6): 383-7.
5. Alvarez EO. The role of histamine on cognition. Behav Brain Res. 2009; 199(2): 183-9.
6. Prell GD, Green JP. Measurement of histamine metabolites in brain and cerebrospinal fluid provides insights into histaminergic activity. Agents Actions. 1994; 41: C5-8.