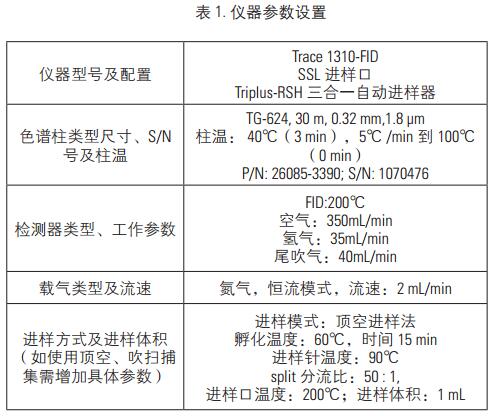
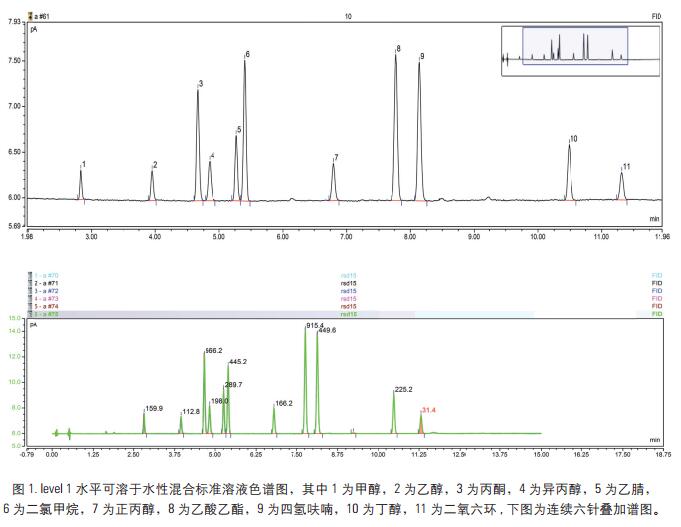
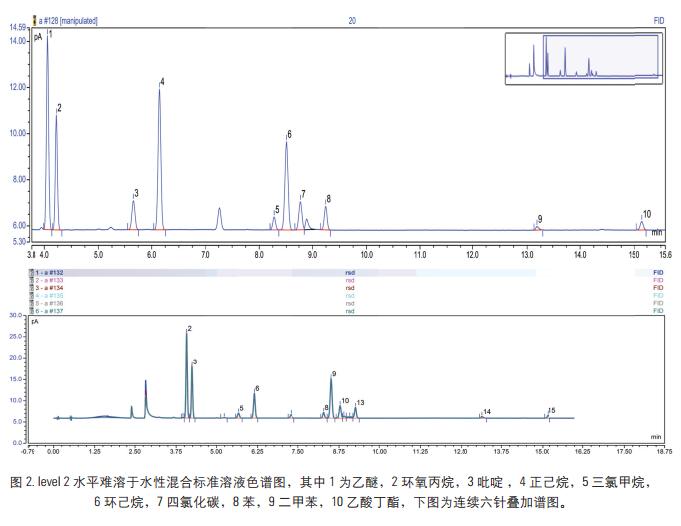
Key words Solvent residue; Triplus-RSH; gas phase aims The method develops a solvent residue in a drug by a gas-tight needle headspace, and determines a solvent residue according to different drug dosage forms. For the drugs in the pharmacopoeia that require the determination of solvent residues, the six-way valve-quantitative ring headspace analysis is used. The method uses gas-tight needle headspace to analyze it. The measured results are accurate and reproducible. Analysis of solvent residues. introduction Since the drug often introduces various residual solvents during the production and storage process, in order to ensure the quality of the drug and the safety of the drug, it is particularly important to control the solvent residue of the drug substance and the drug auxiliary in the production. The guiding principle of the residual solvent determination method in the Appendix 2 of the Chinese Pharmacopoeia of 2010 is mainly to determine several organic solvents by headspace gas chromatography. According to different solubility of different drugs, the method mainly uses two analytical methods to analyze and determine common solvent residues in two categories. The result is accurate, reliable and reproducible. instrument Thermo Scientific TM Dionex TM Chromeleon TM Chromatography Data Reagents and consumables Thermo Scientific TG-624 chromatographic column (30 m × 0.32 mm × 1.8 μm) Preparation of standard solution The concentration of the two groups of solvents is as follows, wherein the poorly soluble in water is dissolved in N,N-dimethylformamide, and the water soluble is water. Low concentration 1.33-7.10 mg/mL, high concentration: 79.8-473.33 mg/mL calibration stock solution, diluted to the following concentrations. Sample Preparation Accurately measure 0.5g of the drug in a 20mL headspace bottle, add 5mL of water or 3mL of N,N-dimethylformamide, shake it to dissolve the drug dissolved in water and add 0.5g of sodium chloride to seal it. The product solution is to be tested. Gas chromatographic conditions Instrument conditions are shown in Table 1. Results and discussion Optimization of headspace conditions According to the different solubility of different drugs, water and N,N-dimethylformamide were selected as the solvent in this experiment, so the headspace temperature is not easy to be too high, so choose 60 °C as the headspace equilibrium temperature at the equilibrium temperature. The response value of each chromatographic peak reaches the maximum at 15 min, that is, the gas-liquid two phases of the headspace have basically reached equilibrium, and the equilibrium time should not be too long. If it is too long, the airtightness of the headspace bottle may be caused. Deterioration results in a decrease in the accuracy of the quantification, which may result in poor sensitivity. Therefore, 15 min was chosen as the headspace equilibration time in this experiment. For this experiment, a gas-tight needle headspace was used. In the experiment, the gas syringe temperature was set to 90 °C, at which temperature the sample was not condensed at the needle, which effectively ensured the integrity of the injection volume. Column selection For solvent-resistance columns, TG-5, TG-1, TG-wax, TG-624, etc. can be selected depending on the analytes. For some samples, the interference peaks are selected to select the column. In addition, for the separation of benzene series, if the method for quantifying xylene is separately selected, a polar polyethylene glycol type column should be selected, and for the method requiring separation of more substances, a TG-624 column is recommended. In the method, all solvent residues are classified into two types according to different solubility drugs, one is water soluble in Figure 1, and the other is water insoluble in Figure 2. The chromatogram of the 21 solvent residues is shown in the figure below. Linearity, detection limit and RSD Prepare the concentration level: the calibration solution at the level 1-5 level, and separately analyze the sample by the above method to investigate the linearity of each component in the concentration range. The experimental results show that the 21 components have a good linear relationship in this range, and the linear correlation coefficients are all greater than 0.99 (see Table 4). Add mixed standard solution (addition concentration level level1, level2) to the drug, and investigate the recovery of the 21 solvent residues. The experimental results show that the recoveries of the components are between 83-100%, which meets the requirements of daily analytical testing. The levels of level1 and level2 were measured in parallel six times in parallel, and the average RSD value was 2.4-8.7%, which met the stability requirement. At the same time, the detection limit of each component was calculated by three times the signal-to-noise ratio, and the detection limit of each component was between 0.2-14μg/mL. Actual sample test Test an unknown drug using established analytical methods. The experimental results show that the method can determine the solvent residue in the drug. In addition, for some drugs, the matrix is ​​more complicated. It is recommended to select the temperature of the final temperature program to a higher level to ensure that there is no pollution to the column. to sum up Using the new generation of TRACE 1310 GC from Thermo Fisher, combined with the Triplus-RSH three-in-one autosampler, this experiment uses the headspace function. It has the advantages of quick and convenient installation, high sensitivity, good repeatability and reliable results. This paper fully meets the needs of solvent residue analysis and detection in the pharmacopoeia, and can easily respond to various VOC analysis requirements in the laboratory. references [1] Zhang Yuhua, Han Xuejing, Zhang Xiru et al. Simultaneous determination of six organic solvent residues in diammonium glycyrrhizinate raw materials by headspace gas chromatography. Chinese Pharmacist, 2012, 15(12): 1713-1715. A Foot Spa Machine with heat is a device used to provide a relaxing and therapeutic foot massage. It usually has a basin filled with warm water and has various massage settings such as vibration, bubbles, and rollers. The heat function helps soothe tired and aching feet, while the massage setting provides a deep-tissue massage that helps improve circulation, reduce tension, and relieve pain. Some foot spas also come with removable attachments, such as pumice stones and brushes, for extra exfoliation and cleansing. Overall, a foot spa machine with heat is a great tool for anyone looking to pamper their feet and promote overall relaxation and wellness. Foot Spa Machine With Heat,Bubble Foot Bath Massager,Foot Massage Machine,Pedicure Foot Spa Machine Huaian Mimir Electric Appliance Co., LTD , https://www.mmfootbath.com
System software, version 7.1
Thermo Scientific Trace 1310 Gas Chromatography with FID
Thermo Scientific Triplus RSH auto-sampler;
21 solvent residue standards, purchased from National Standards Center and Accustandard; N,N-dimethylformamide (chromatographically pure, Thermo Fisher Scientific); sodium chloride (analytical grade, Beijing Chemical Reagent Factory) 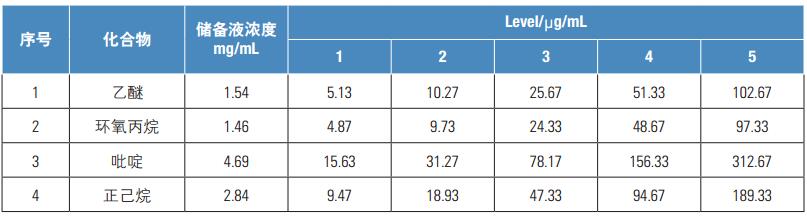

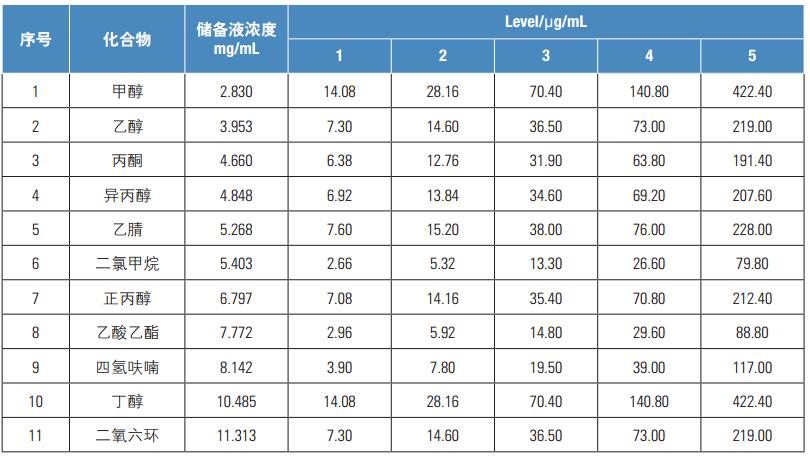




[2] Zhang Yahong, Mi Yazhen. Simultaneous Determination of Nine Residual Solvents in Drug Packaging Composite Films by Headspace Gas Chromatography[J]. Tianjin Pharmaceutical Journal, 2013, 25(5): 48-50.
[3] 2010 edition of the Chinese Pharmacopoeia [S].