We're Professional Supplier Extract Powder manufacturers and suppliers in China specialized in providing high-quality products at low price. We warmly welcome you to buy or wholesale bulk Supplier Extract Powder for sale here from our factory. For a free sample, contact us now.
Supplier Extract Powder,Supplier Extract ,Supplier Powder Manufacturer in China Shaanxi Kang New Pharmaceutical co., Ltd. , https://www.kangnewpharmas.com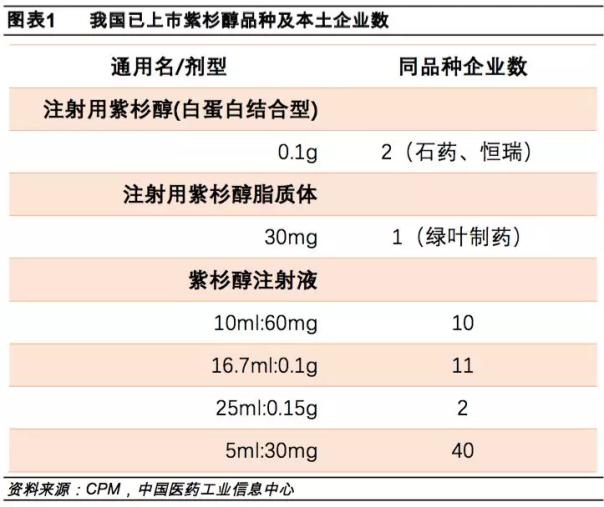
Haizheng paclitaxel (albumin binding type) is coming! Third evaluation of consistency
Medical Network November 28th According to the China New Drug Development Monitoring Database (CPM), Haizheng Pharmaceutical submitted the injection paclitaxel (albumin-binding type) "chemical imitation application for production" changed to the acceptance status.
This is the third injectable paclitaxel (albumin-binding) company that has been listed in the new registration of chemical drugs in China after Shiyan and Hengrui. If passed, it will be directly included in the "China Listed Drug List" and the "Certificate of Conformity" logo can be used to indicate that the drug is consistent with the quality and efficacy of the original drug.
Speaking of paclitaxel, it’s probably no one knows no one. It is the best natural anticancer drug that has been discovered so far. BMS was approved by the FDA on December 29, 1992. Once it was listed, the sales volume in the next year was successfully broken by 100 million US dollars. In 1998, it broke through the $1 billion mark. Bombshell-class products. Later, with the introduction of the dominant dosage forms and similar products, the sales of paclitaxel in BMS gradually declined.
At present, the domestically marketed paclitaxel varieties mainly include paclitaxel injection, paclitaxel liposome injection, and paclitaxel for injection (albumin binding type).
Paclitaxel (albumin-binding) was developed by Abraxis in the United States (later acquired by Celgene) to optimize the improved dosage form, which has better curative effect, superior water solubility and lowest toxic side effects than traditional dosage forms. Approved by the FDA in 2005 and approved by the CFDA in 2013, the trade name is Abraxane.
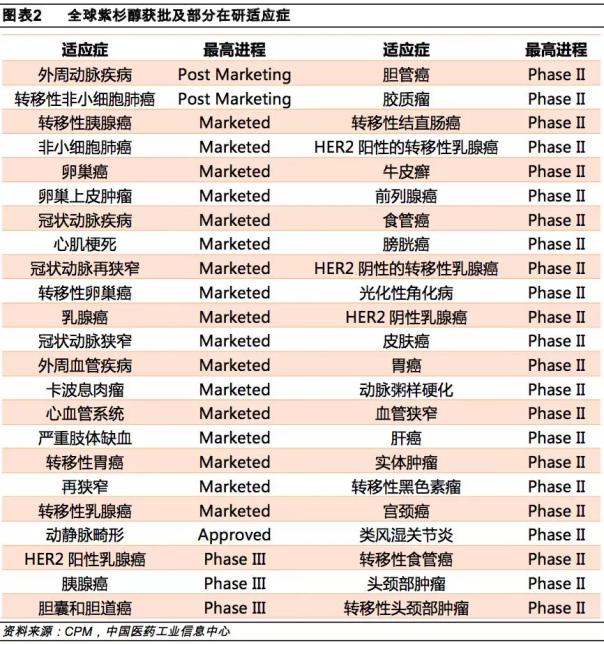
Due to the cytotoxic and anti- tumor principle of paclitaxel, it has a good chemotherapy effect in many tumors, and its indications will continue to expand. Up to now, the indications for paclitaxel approval have been extended to 20, and there are still 26 indications for clinical II and III.
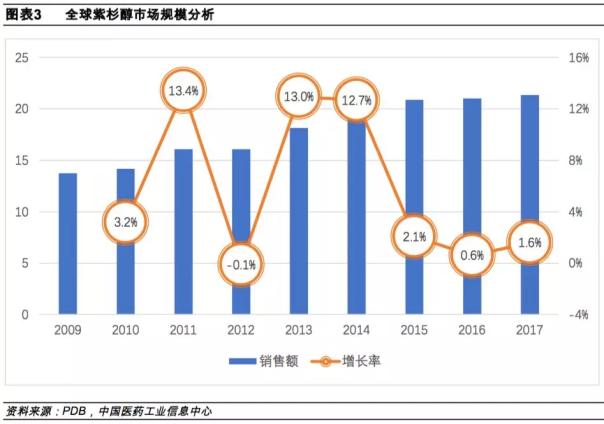
According to the Comprehensive Drug Database (PDB), the market size of paclitaxel in the global market in 2017 has reached 2.135 billion US dollars, with a compound annual growth rate of 5.6%.
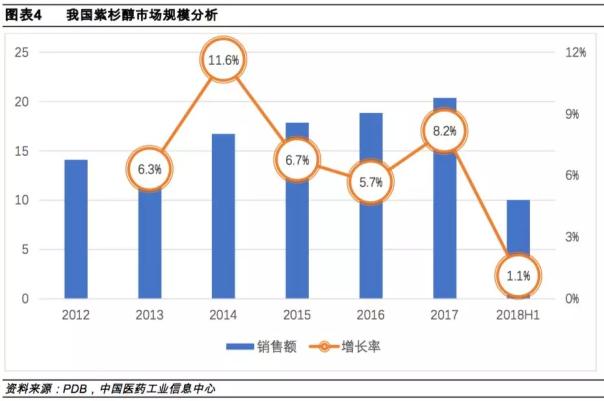
In the domestic market, the sales of paclitaxel have been increasing for five consecutive years. In 2017, the market size of sample hospitals exceeded 2 billion yuan, ranking the fifth in domestic pharmaceutical sales. The first four varieties were sodium chloride ( Medicinal), human albumin, clopidogrel, atorvastatin.
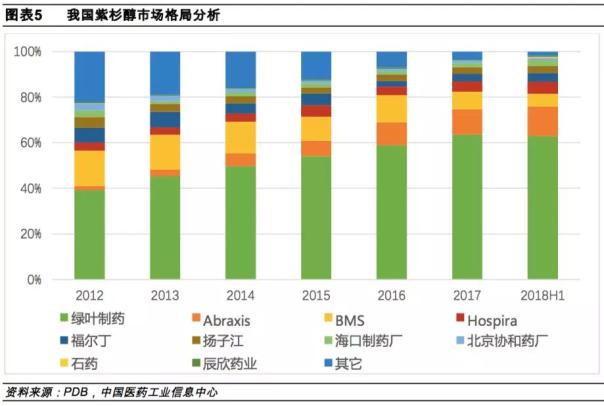
In addition, in the market pattern, paclitaxel market concentration is high, in the 2018 H1 sales TOP5 market share of more than 90%, of which green leaf pharmaceuticals accounted for the highest proportion, followed by Abraxis and BMS.
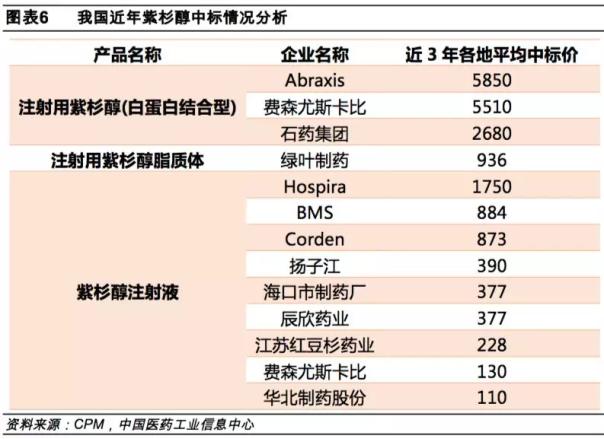
The original research Abraxane has not been released in the past five years, which is related to its high market pricing. Abraxane is about 5,850 yuan per bottle, which is the highest price among similar products. (*Hengrui was approved on August 27th and no winning bid data)
The sea is medicine paclitaxel (albumin-bound) to declare the listing if adopted, would become the third of the approved product conformity evaluation of manufacturer. That is to say, paclitaxel (albumin-binding type) is also full of three. After breaking the import monopoly in February, the paclitaxel (albumin-binding) market has ushered in a severe test. It is expected that the price of this product will soon face a cliff. Fall.